A multiprotein nuclear complex connects Fanconi anemia and Bloom syndrome
- PMID: 12724401
- PMCID: PMC164758
- DOI: 10.1128/MCB.23.10.3417-3426.2003
A multiprotein nuclear complex connects Fanconi anemia and Bloom syndrome
Abstract
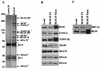
Bloom syndrome (BS) is a genetic disorder associated with dwarfism, immunodeficiency, reduced fertility, and an elevated risk of cancer. To investigate the mechanism of this disease, we isolated from human HeLa extracts three complexes containing the helicase defective in BS, BLM. Interestingly, one of the complexes, termed BRAFT, also contains five of the Fanconi anemia (FA) complementation group proteins (FA proteins). FA resembles BS in genomic instability and cancer predisposition, but most of its gene products have no known biochemical activity, and the molecular pathogenesis of the disease is poorly understood. BRAFT displays a DNA-unwinding activity, which requires the presence of BLM because complexes isolated from BLM-deficient cells lack such an activity. The complex also contains topoisomerase IIIalpha and replication protein A, proteins that are known to interact with BLM and could facilitate unwinding of DNA. We show that BLM complexes isolated from an FA cell line have a lower molecular mass. Our study provides the first biochemical characterization of a multiprotein FA complex and suggests a connection between the BLM and FA pathways of genomic maintenance. The findings that FA proteins are part of a DNA-unwinding complex imply that FA proteins may participate in DNA repair.
Figures
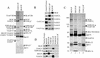
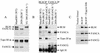




Similar articles
-
FANCM connects the genome instability disorders Bloom's Syndrome and Fanconi Anemia.Mol Cell. 2009 Dec 25;36(6):943-53. doi: 10.1016/j.molcel.2009.12.006. Mol Cell. 2009. PMID: 20064461
-
Interaction between the helicases genetically linked to Fanconi anemia group J and Bloom's syndrome.EMBO J. 2011 Feb 16;30(4):692-705. doi: 10.1038/emboj.2010.362. Epub 2011 Jan 14. EMBO J. 2011. PMID: 21240188 Free PMC article.
-
Topo IIIalpha and BLM act within the Fanconi anemia pathway in response to DNA-crosslinking agents.Cytogenet Genome Res. 2009;125(3):165-75. doi: 10.1159/000230001. Epub 2009 Sep 4. Cytogenet Genome Res. 2009. PMID: 19738377 Free PMC article.
-
Identification and analysis of new proteins involved in the DNA damage response network of Fanconi anemia and Bloom syndrome.Methods. 2009 May;48(1):72-9. doi: 10.1016/j.ymeth.2009.02.011. Epub 2009 Feb 24. Methods. 2009. PMID: 19245838 Free PMC article. Review.
-
Fanconi anaemia proteins are associated with sister chromatid bridging in mitosis.Int J Hematol. 2011 Apr;93(4):440-445. doi: 10.1007/s12185-011-0818-7. Epub 2011 Apr 8. Int J Hematol. 2011. PMID: 21472397 Review.
Cited by
-
FANCD2 regulates BLM complex functions independently of FANCI to promote replication fork recovery.Nucleic Acids Res. 2013 Jul;41(13):6444-59. doi: 10.1093/nar/gkt348. Epub 2013 May 8. Nucleic Acids Res. 2013. PMID: 23658231 Free PMC article.
-
Histone H2AX and Fanconi anemia FANCD2 function in the same pathway to maintain chromosome stability.EMBO J. 2007 Mar 7;26(5):1340-51. doi: 10.1038/sj.emboj.7601574. Epub 2007 Feb 15. EMBO J. 2007. PMID: 17304220 Free PMC article.
-
UBE2T, the Fanconi anemia core complex, and FANCD2 are recruited independently to chromatin: a basis for the regulation of FANCD2 monoubiquitination.Mol Cell Biol. 2007 Dec;27(24):8421-30. doi: 10.1128/MCB.00504-07. Epub 2007 Oct 15. Mol Cell Biol. 2007. PMID: 17938197 Free PMC article.
-
FANCJ (BACH1) helicase forms DNA damage inducible foci with replication protein A and interacts physically and functionally with the single-stranded DNA-binding protein.Blood. 2007 Oct 1;110(7):2390-8. doi: 10.1182/blood-2006-11-057273. Epub 2007 Jun 27. Blood. 2007. PMID: 17596542 Free PMC article.
-
Inflammatory ROS promote and cooperate with the Fanconi anemia mutation for hematopoietic senescence.J Cell Sci. 2007 May 1;120(Pt 9):1572-83. doi: 10.1242/jcs.003152. Epub 2007 Apr 3. J Cell Sci. 2007. PMID: 17405815 Free PMC article.
References
-
- Bronner, C. E., S. M. Baker, P. T. Morrison, G. Warren, L. G. Smith, M. K. Lescoe, M. Kane, C. Earabino, J. Lipford, A. Lindblom, et al. 1994. Mutation in the DNA mismatch repair gene homologue hMLH1 is associated with hereditary non-polyposis colon cancer. Nature 368:258-261. - PubMed
-
- Brosh, R. M., Jr., J. L. Li, M. K. Kenny, J. K. Karow, M. P. Cooper, R. P. Kureekattil, I. D. Hickson, and V. A. Bohr. 2000. Replication protein A physically interacts with the Bloom's syndrome protein and stimulates its helicase activity. J. Biol. Chem. 275:23500-23508. - PubMed
-
- Brosh, R. M., Jr., A. Majumdar, S. Desai, I. D. Hickson, V. A. Bohr, and M. M. Seidman. 2001. Unwinding of a DNA triple helix by the Werner and Bloom syndrome helicases. J. Biol. Chem. 276:3024-3030. - PubMed
-
- Cumming, R. C., J. Lightfoot, K. Beard, H. Youssoufian, P. J. O'Brien, and M. Buchwald. 2001. Fanconi anemia group C protein prevents apoptosis in hematopoietic cells through redox regulation of GSTP1. Nat. Med. 7:814-820. - PubMed
-
- D'Andrea, A. D. 2001. Cellular function of the Fanconi anemia pathway. Nat. Med. 7:1259-1260. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous
