Vascular defects and sensorineural deafness in a mouse model of Norrie disease
- PMID: 12040033
- PMCID: PMC6758776
- DOI: 10.1523/JNEUROSCI.22-11-04286.2002
Vascular defects and sensorineural deafness in a mouse model of Norrie disease
Abstract
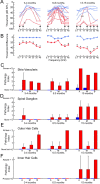
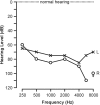
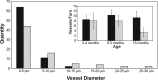
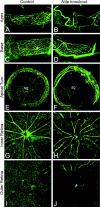
Norrie disease is an X-linked recessive syndrome of blindness, deafness, and mental retardation. A knock-out mouse model with an Ndp gene disruption was studied. We examined the hearing phenotype, including audiological, histological, and vascular evaluations. As is seen in humans, the mice had progressive hearing loss leading to profound deafness. The primary lesion was localized to the stria vascularis, which houses the main vasculature of the cochlea. Fluorescent dyes showed an abnormal vasculature in this region and eventual loss of two-thirds of the vessels. We propose that one of the principal functions of norrin in the ear is to regulate the interaction of the cochlea with its vasculature.
Figures


Similar articles
-
Noncoding Microdeletion in Mouse Hgf Disrupts Neural Crest Migration into the Stria Vascularis, Reduces the Endocochlear Potential, and Suggests the Neuropathology for Human Nonsyndromic Deafness DFNB39.J Neurosci. 2020 Apr 8;40(15):2976-2992. doi: 10.1523/JNEUROSCI.2278-19.2020. Epub 2020 Mar 9. J Neurosci. 2020. PMID: 32152201 Free PMC article.
-
Audiologic features of Norrie disease.Ann Otol Rhinol Laryngol. 2005 Jul;114(7):533-8. doi: 10.1177/000348940511400707. Ann Otol Rhinol Laryngol. 2005. PMID: 16134349
-
Hearing and vestibular deficits in the Coch(-/-) null mouse model: comparison to the Coch(G88E/G88E) mouse and to DFNA9 hearing and balance disorder.Hear Res. 2011 Feb;272(1-2):42-8. doi: 10.1016/j.heares.2010.11.002. Epub 2010 Nov 10. Hear Res. 2011. PMID: 21073934 Free PMC article.
-
Hearing loss in the RBF/DnJ mouse, a proposed animal model of Usher syndrome type IIa.Hear Res. 1997 Oct;112(1-2):1-12. doi: 10.1016/s0378-5955(97)00087-7. Hear Res. 1997. PMID: 9367224
-
Cochlear histopathology in human genetic hearing loss: State of the science and future prospects.Hear Res. 2019 Oct;382:107785. doi: 10.1016/j.heares.2019.107785. Epub 2019 Aug 19. Hear Res. 2019. PMID: 31493568 Free PMC article. Review.
Cited by
-
Transgenic Tg(Kcnj10-ZsGreen) fluorescent reporter mice allow visualization of intermediate cells in the stria vascularis.Sci Rep. 2024 Feb 6;14(1):3038. doi: 10.1038/s41598-024-52663-7. Sci Rep. 2024. PMID: 38321040 Free PMC article.
-
Norrin, frizzled-4, and Lrp5 signaling in endothelial cells controls a genetic program for retinal vascularization.Cell. 2009 Oct 16;139(2):285-98. doi: 10.1016/j.cell.2009.07.047. Cell. 2009. PMID: 19837032 Free PMC article.
-
Immunocytochemical distribution of WARP (von Willebrand A domain-related protein) in the inner ear.Brain Res. 2011 Jan 7;1367:50-61. doi: 10.1016/j.brainres.2010.10.056. Epub 2010 Nov 18. Brain Res. 2011. PMID: 20971096 Free PMC article.
-
Transgenic Tg(Kcnj10-ZsGreen) Fluorescent Reporter Mice Allow Visualization of Intermediate Cells in the Stria Vascularis.Res Sq [Preprint]. 2023 Oct 4:rs.3.rs-3393161. doi: 10.21203/rs.3.rs-3393161/v1. Res Sq. 2023. Update in: Sci Rep. 2024 Feb 6;14(1):3038. doi: 10.1038/s41598-024-52663-7. PMID: 37886521 Free PMC article. Updated. Preprint.
-
Assessment of Inner Blood-Retinal Barrier: Animal Models and Methods.Cells. 2023 Oct 12;12(20):2443. doi: 10.3390/cells12202443. Cells. 2023. PMID: 37887287 Free PMC article. Review.
References
-
- Ando M, Takeuchi S. Postnatal vascular development in the lateral wall of the cochlear duct of gerbils: quantitative analysis by electron microscopy and confocal laser microscopy. Hear Res. 1998;123:148–156. - PubMed
-
- Anniko M, Bagger-Sjoback D. The stria vascularis. In: Friedmann I, Ballantyne J, editors. Ultrastructural atlas of the inner ear. Butterworths; Boston: 1984. p. 329.
-
- Berger W, Meindl A, van de Pol TJ, Cremers FP, Ropers HH, Doerner C, Monaco A, Bergen AA, Lebo R, Warburg M, Zergollern L, Lorenz B, Gal A, Bleeker-Wagemakers EM, Meitinger T. Isolation of a candidate gene for Norrie disease by positional cloning. Nat Genet. 1992;1:199–203. - PubMed
-
- Berger W, van de Pol D, Bachner D, Oerlemans F, Winkens H, Hameister H, Wieringa B, Hendriks W, Ropers HH. An animal model for Norrie disease (ND): gene targeting of the mouse ND gene. Hum Mol Genet. 1996;5:51–59. - PubMed
-
- Chen ZY, Hendriks RW, Jobling MA, Powell JF, Breakefield XO, Sims KB, Craig IW. Isolation and characterization of a candidate gene for Norrie disease. Nat Genet. 1992;1:204–208. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
