Complement activation in factor D-deficient mice
- PMID: 11724962
- PMCID: PMC64724
- DOI: 10.1073/pnas.261428398
Complement activation in factor D-deficient mice
Abstract
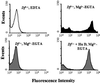
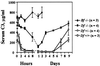
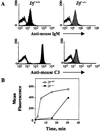
To assess the contribution of the alternative pathway in complement activation and host defense and its possible role in the regulation of systemic energy balance in vivo, factor D-deficient mice were generated by gene targeting. The mutant mice have no apparent abnormality in development and their body weights are similar to those of factor D-sufficient littermates. Complement activation could not be initiated in the serum of deficient mice by the alternative pathway activators rabbit erythrocytes and zymosan. Surprisingly, injection of cobra venom factor (CVF) caused a profound and reproducible reduction in serum C3 levels, whereas, as expected, there was no C3 reduction in factor B-deficient mice treated similarly. Studies of C3 and factor B activation in vitro by CVF demonstrated that in factor D-deficient serum the alpha chain of C3 was cleaved gradually over a period of 60 min without detectable cleavage of factor B. CVF-dependent C3 cleavage in the deficient serum required the presence of Mg(2+), whereas in normal mouse serum the presence of divalent cations was not required. These results suggest that in mouse proteolytic cleavage of factor B by factor D is not an absolute requirement for the zymogen to active enzyme conformational transition of CVF-bound factor B. Kinetics of opsonization of Streptococcus pneumoniae by C3 fragments was much slower in factor D-deficient serum, suggesting a significant contribution of the alternative pathway to antibacterial host defense early after infection.
Figures
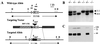

Similar articles
-
Complement 1 inhibitor is a regulator of the alternative complement pathway.J Exp Med. 2001 Dec 3;194(11):1609-16. doi: 10.1084/jem.194.11.1609. J Exp Med. 2001. PMID: 11733575 Free PMC article.
-
Interaction of desialated guinea pig erythrocytes with the classical and alternative pathways of guinea pig complement in vivo and in vitro.J Clin Invest. 1983 Jun;71(6):1710-9. doi: 10.1172/jci110925. J Clin Invest. 1983. PMID: 6863540 Free PMC article.
-
The alternative activation pathway and complement component C3 are critical for a protective immune response against Pseudomonas aeruginosa in a murine model of pneumonia.Infect Immun. 2004 May;72(5):2899-906. doi: 10.1128/IAI.72.5.2899-2906.2004. Infect Immun. 2004. PMID: 15102802 Free PMC article.
-
The cobra complement system: I. The alternative pathway of activation.Dev Comp Immunol. 1985 Spring;9(2):311-25. doi: 10.1016/0145-305x(85)90122-3. Dev Comp Immunol. 1985. PMID: 3894085 Review.
-
Complement abnormalities in multiple myeloma.Am J Med. 1989 Oct;87(4):411-20. doi: 10.1016/s0002-9343(89)80824-1. Am J Med. 1989. PMID: 2679074 Review.
Cited by
-
The role of complement component 3 (C3) in differentiation of myeloid-derived suppressor cells.Blood. 2013 Mar 7;121(10):1760-8. doi: 10.1182/blood-2012-06-440214. Epub 2013 Jan 8. Blood. 2013. PMID: 23299310 Free PMC article.
-
Higher Adherence to a Mediterranean Diet Is Associated with Improved Insulin Sensitivity and Selected Markers of Inflammation in Individuals Who Are Overweight and Obese without Diabetes.Nutrients. 2022 Oct 21;14(20):4437. doi: 10.3390/nu14204437. Nutrients. 2022. PMID: 36297122 Free PMC article.
-
Mannan-Binding Lectin-Associated Serine Protease 1/3 Cleavage of Pro-Factor D into Factor D In Vivo and Attenuation of Collagen Antibody-Induced Arthritis through Their Targeted Inhibition by RNA Interference-Mediated Gene Silencing.J Immunol. 2016 Nov 1;197(9):3680-3694. doi: 10.4049/jimmunol.1600719. Epub 2016 Oct 5. J Immunol. 2016. PMID: 27707997 Free PMC article.
-
Increased serum C3 levels in Crry transgenic mice partially abrogates its complement inhibitory effects.Clin Exp Immunol. 2004 May;136(2):194-9. doi: 10.1111/j.1365-2249.2004.02450.x. Clin Exp Immunol. 2004. PMID: 15086380 Free PMC article.
-
Enhanced susceptibility to acute pneumococcal otitis media in mice deficient in complement C1qa, factor B, and factor B/C2.Infect Immun. 2010 Mar;78(3):976-83. doi: 10.1128/IAI.01012-09. Epub 2010 Jan 11. Infect Immun. 2010. PMID: 20065024 Free PMC article.
References
-
- Volanakis J E. In: The Human Complement System in Health and Disease. Volanakis J E, Frank M M, editors. New York: Dekker; 1998. pp. 9–32.
-
- Flier J S, Cook K S, Usher P, Spiegelman B M. Science. 1987;237:405–408. - PubMed
-
- Rosen B S, Cook K S, Yaglom J, Groves D L, Volanakis J E, Damm D, White T, Spiegelman B M. Science. 1989;244:1483–1487. - PubMed
-
- Choy L N, Rosen B S, Spiegelman B M. J Biol Chem. 1992;267:12736–12741. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

