Transcription-independent RNA polymerase II dephosphorylation by the FCP1 carboxy-terminal domain phosphatase in Xenopus laevis early embryos
- PMID: 11533226
- PMCID: PMC99784
- DOI: 10.1128/MCB.21.19.6359-6368.2001
Transcription-independent RNA polymerase II dephosphorylation by the FCP1 carboxy-terminal domain phosphatase in Xenopus laevis early embryos
Abstract
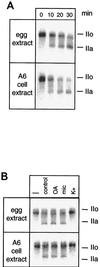
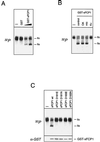
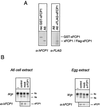
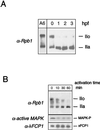
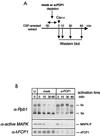
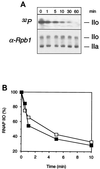
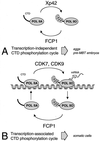
The phosphorylation of the RNA polymerase II (RNAP II) carboxy-terminal domain (CTD) plays a key role in mRNA metabolism. The relative ratio of hyperphosphorylated RNAP II to hypophosphorylated RNAP II is determined by a dynamic equilibrium between CTD kinases and CTD phosphatase(s). The CTD is heavily phosphorylated in meiotic Xenopus laevis oocytes. In this report we show that the CTD undergoes fast and massive dephosphorylation upon fertilization. A cDNA was cloned and shown to code for a full-length xFCP1, the Xenopus orthologue of the FCP1 CTD phosphatases in humans and Saccharomyces cerevisiae. Two critical residues in the catalytic site were identified. CTD phosphatase activity was observed in extracts prepared from Xenopus eggs and cells and was shown to be entirely attributable to xFCP1. The CTD dephosphorylation triggered by fertilization was reproduced upon calcium activation of cytostatic factor-arrested egg extracts. Using immunodepleted extracts, we showed that this dephosphorylation is due to xFCP1. Although transcription does not occur at this stage, phosphorylation appears as a highly dynamic process involving the antagonist action of Xp42 mitogen-activated protein kinase and FCP1 phosphatase. This is the first report that free RNAP II is a substrate for FCP1 in vivo, independent from a transcription cycle.
Figures

Similar articles
-
FCP1 phosphorylation by casein kinase 2 enhances binding to TFIIF and RNA polymerase II carboxyl-terminal domain phosphatase activity.J Biol Chem. 2002 Sep 27;277(39):36061-7. doi: 10.1074/jbc.M205192200. Epub 2002 Jul 22. J Biol Chem. 2002. PMID: 12138108
-
The RNA Pol II CTD phosphatase Fcp1 is essential for normal development in Drosophila melanogaster.Gene. 2009 Oct 15;446(2):58-67. doi: 10.1016/j.gene.2009.07.012. Epub 2009 Jul 24. Gene. 2009. PMID: 19632310
-
Dephosphorylation of RNA polymerase II by CTD-phosphatase FCP1 is inhibited by phospho-CTD associating proteins.J Mol Biol. 2004 Jan 9;335(2):415-24. doi: 10.1016/j.jmb.2003.10.036. J Mol Biol. 2004. PMID: 14672652
-
CTD phosphatase: role in RNA polymerase II cycling and the regulation of transcript elongation.Prog Nucleic Acid Res Mol Biol. 2002;72:333-65. doi: 10.1016/s0079-6603(02)72074-6. Prog Nucleic Acid Res Mol Biol. 2002. PMID: 12206456 Review.
-
Regulation of RNA polymerase II activity by CTD phosphorylation and cell cycle control.J Cell Physiol. 2002 Feb;190(2):160-9. doi: 10.1002/jcp.10058. J Cell Physiol. 2002. PMID: 11807820 Review.
Cited by
-
Arabidopsis C-terminal domain phosphatase-like 1 and 2 are essential Ser-5-specific C-terminal domain phosphatases.Proc Natl Acad Sci U S A. 2004 Oct 5;101(40):14539-44. doi: 10.1073/pnas.0403174101. Epub 2004 Sep 23. Proc Natl Acad Sci U S A. 2004. PMID: 15388846 Free PMC article.
-
Genetic interactions between an RNA polymerase II phosphatase and centromeric elements in Saccharomyces cerevisiae.Mol Genet Genomics. 2004 Jun;271(5):603-15. doi: 10.1007/s00438-004-1009-5. Epub 2004 May 6. Mol Genet Genomics. 2004. PMID: 15133655
-
Transcription reactivation steps stimulated by oocyte maturation in C. elegans.Dev Biol. 2007 Apr 1;304(1):382-93. doi: 10.1016/j.ydbio.2006.12.039. Epub 2006 Dec 23. Dev Biol. 2007. PMID: 17291483 Free PMC article.
-
The FCP1 phosphatase interacts with RNA polymerase II and with MEP50 a component of the methylosome complex involved in the assembly of snRNP.Nucleic Acids Res. 2003 Feb 1;31(3):999-1005. doi: 10.1093/nar/gkg197. Nucleic Acids Res. 2003. PMID: 12560496 Free PMC article.
-
The C-terminal domain phosphatase and transcription elongation activities of FCP1 are regulated by phosphorylation.Proc Natl Acad Sci U S A. 2003 Mar 4;100(5):2328-33. doi: 10.1073/pnas.2628049100. Epub 2003 Feb 18. Proc Natl Acad Sci U S A. 2003. PMID: 12591939 Free PMC article.
References
-
- Archambault J, Pan G, Dahmus G K, Cartier M, Marshall N F, Zhang S, Dahmus M E, Greenblatt J. FCP1, the RAP74-interacting subunit of a human protein phosphatase that dephosphorylates the carboxyl-terminal domain of RNA polymerase IIO. J Biol Chem. 1998;273:27593–27601. - PubMed
-
- Bensaude O, Bonnet F, Cassé C, Dubois M-F, Nguyen V-T, Palancade B. Regulated phosphorylation of the RNA polymerase II C-terminal domain (CTD) Biochem Cell Biol. 1999;77:1–7. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
