Crystal structure of isopentenyl diphosphate:dimethylallyl diphosphate isomerase
- PMID: 11285217
- PMCID: PMC145486
- DOI: 10.1093/emboj/20.7.1530
Crystal structure of isopentenyl diphosphate:dimethylallyl diphosphate isomerase
Abstract
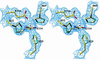
Isopentenyl diphosphate:dimethylallyl diphosphate (IPP:DMAPP) isomerase catalyses a crucial activation step in the isoprenoid biosynthesis pathway. This enzyme is responsible for the isomerization of the carbon-carbon double bond of IPP to create the potent electrophile DMAPP. DMAPP then alkylates other molecules, including IPP, to initiate the extraordinary variety of isoprenoid compounds found in nature. The crystal structures of free and metal-bound Escherichia coli IPP isomerase reveal critical active site features underlying its catalytic mechanism. The enzyme requires one Mn(2+) or Mg(2+) ion to fold in its active conformation, forming a distorted octahedral metal coordination site composed of three histidines and two glutamates and located in the active site. Two critical residues, C67 and E116, face each other within the active site, close to the metal-binding site. The structures are compatible with a mechanism in which the cysteine initiates the reaction by protonating the carbon-carbon double bond, with the antarafacial rearrangement ultimately achieved by one of the glutamates involved in the metal coordination sphere. W161 may stabilize the highly reactive carbocation generated during the reaction through quadrupole- charge interaction.
Figures
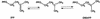


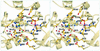


Similar articles
-
The crystal structure of human isopentenyl diphosphate isomerase at 1.7 A resolution reveals its catalytic mechanism in isoprenoid biosynthesis.J Mol Biol. 2007 Mar 9;366(5):1447-58. doi: 10.1016/j.jmb.2006.12.055. Epub 2006 Dec 24. J Mol Biol. 2007. PMID: 17250851
-
Escherichia coli type I isopentenyl diphosphate isomerase: structural and catalytic roles for divalent metals.J Am Chem Soc. 2006 Sep 6;128(35):11545-50. doi: 10.1021/ja063073c. J Am Chem Soc. 2006. PMID: 16939278
-
Crystal structures of human IPP isomerase: new insights into the catalytic mechanism.J Mol Biol. 2007 Mar 9;366(5):1437-46. doi: 10.1016/j.jmb.2006.10.092. Epub 2006 Nov 3. J Mol Biol. 2007. PMID: 17137593
-
Isopentenyl diphosphate isomerase: A checkpoint to isoprenoid biosynthesis.Biochimie. 2012 Aug;94(8):1621-34. doi: 10.1016/j.biochi.2012.03.021. Epub 2012 Apr 4. Biochimie. 2012. PMID: 22503704 Review.
-
Perspectives in anti-infective drug design. The late steps in the biosynthesis of the universal terpenoid precursors, isopentenyl diphosphate and dimethylallyl diphosphate.Bioorg Chem. 2004 Oct;32(5):292-308. doi: 10.1016/j.bioorg.2004.05.012. Bioorg Chem. 2004. PMID: 15381396 Review.
Cited by
-
Isopentenyl diphosphate isomerase catalyzed reactions in D2O: product release limits the rate of this sluggish enzyme-catalyzed reaction.J Am Chem Soc. 2012 Apr 18;134(15):6568-70. doi: 10.1021/ja302154k. Epub 2012 Apr 5. J Am Chem Soc. 2012. PMID: 22471428 Free PMC article.
-
Molecular docking as a popular tool in drug design, an in silico travel.Adv Appl Bioinform Chem. 2016 Jun 28;9:1-11. doi: 10.2147/AABC.S105289. eCollection 2016. Adv Appl Bioinform Chem. 2016. PMID: 27390530 Free PMC article. Review.
-
Transcriptome and metabolite analyses reveal the complex metabolic genes involved in volatile terpenoid biosynthesis in garden sage (Salvia officinalis).Sci Rep. 2017 Nov 22;7(1):16074. doi: 10.1038/s41598-017-15478-3. Sci Rep. 2017. PMID: 29167468 Free PMC article.
-
Isopentenyl-diphosphate isomerases in human and mouse: evolutionary analysis of a mammalian gene duplication.J Mol Evol. 2003 Sep;57(3):282-91. doi: 10.1007/s00239-003-2476-8. J Mol Evol. 2003. PMID: 14629038
-
Crystal structure of type 2 isopentenyl diphosphate isomerase from Thermus thermophilus in complex with inorganic pyrophosphate.Biochemistry. 2008 Sep 2;47(35):9051-3. doi: 10.1021/bi801159x. Epub 2008 Aug 12. Biochemistry. 2008. PMID: 18693754 Free PMC article.
References
-
- Agranoff B.W., Eggerer,H., Henning,U. and Lynen,F. (1960) Biosynthesis of terpenes. VII. Isopentenyl pyrophosphate isomerase. J. Biol. Chem., 235, 326–332. - PubMed
-
- Anderson M.S., Muehlbacher,M., Street,I.P., Proffitt,J. and Poulter,C.D. (1989) Isopentenyl diphosphate:dimethylallyl diphosphate isomerase. An improved purification of the enzyme and isolation of the gene from Saccharomyces cerevisiae. J. Biol. Chem., 264, 19169–19175. - PubMed
-
- Ashby M.N. and Edwards,P.A. (1990) Elucidation of the deficiency in two yeast coenzyme Q mutants: characterization of the structural gene encoding hexaprenyl pyrophosphate synthetase. J. Biol. Chem., 265, 13157–13164. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

