Male germ cells and photoreceptors, both dependent on close cell-cell interactions, degenerate upon ClC-2 Cl(-) channel disruption
- PMID: 11250895
- PMCID: PMC145530
- DOI: 10.1093/emboj/20.6.1289
Male germ cells and photoreceptors, both dependent on close cell-cell interactions, degenerate upon ClC-2 Cl(-) channel disruption
Abstract
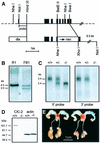
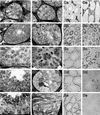
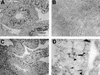
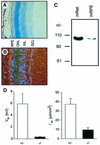
The functions of some CLC Cl(-) channels are evident from human diseases that result from their mutations, but the role of the broadly expressed ClC-2 Cl(-) channel is less clear. Several important functions have been attributed to ClC-2, but contrary to these expectations ClC-2-deficient mice lacked overt abnormalities except for a severe degeneration of the retina and the testes, which led to selective male infertility. Seminiferous tubules did not develop lumina and germ cells failed to complete meiosis. Beginning around puberty there was a massive death of primary spermatocytes and later also of spermatogonia. Tubules were filled with abnormal Sertoli cells, which normally express ClC-2 in patches adjacent to germ cells. In the retina, photoreceptors lacked normal outer segments and degenerated between days P10 and P30. The current across the retinal pigment epithelium was severely reduced at P36. Thus, ClC-2 disruption entails the death of two cell types which depend on supporting cells that form the blood-testes and blood-retina barriers. We propose that ClC-2 is crucial for controlling the ionic environment of these cells.
Figures



Similar articles
-
Severe retinal degeneration associated with disruption of semaphorin 4A.Invest Ophthalmol Vis Sci. 2004 Aug;45(8):2767-77. doi: 10.1167/iovs.04-0020. Invest Ophthalmol Vis Sci. 2004. PMID: 15277503
-
Looking chloride channels straight in the eye: bestrophins, lipofuscinosis, and retinal degeneration.Physiology (Bethesda). 2005 Oct;20:292-302. doi: 10.1152/physiol.00021.2005. Physiology (Bethesda). 2005. PMID: 16174869 Review.
-
Retinal degeneration following failed photoreceptor maturation in 5A11/basigin null mice.Exp Eye Res. 2001 Apr;72(4):467-77. doi: 10.1006/exer.2000.0974. Exp Eye Res. 2001. PMID: 11273674
-
Expression of vascular endothelial growth factor receptors during male germ cell differentiation in the mouse.Biol Reprod. 2003 Sep;69(3):985-94. doi: 10.1095/biolreprod.102.013581. Epub 2003 May 28. Biol Reprod. 2003. PMID: 12773425
-
Expression and function of CLC and cystic fibrosis transmembrane conductance regulator chloride channels in renal epithelial tubule cells: pathophysiological implications.Chang Gung Med J. 2007 Jan-Feb;30(1):17-25. Chang Gung Med J. 2007. PMID: 17477025 Review.
Cited by
-
Apical CLC-2 in retinal pigment epithelium is crucial for survival of the outer retina.FASEB J. 2021 Jul;35(7):e21689. doi: 10.1096/fj.202100349R. FASEB J. 2021. PMID: 34085737 Free PMC article.
-
Case report: A frameshift mutation in CLCN2-related leukoencephalopathy and retinopathy.Front Genet. 2023 Nov 9;14:1278961. doi: 10.3389/fgene.2023.1278961. eCollection 2023. Front Genet. 2023. PMID: 38028614 Free PMC article.
-
Elemental characterization of oral cavity squamous cell carcinoma and its relationship with smoking, prognosis and survival.Sci Rep. 2020 Jun 25;10(1):10382. doi: 10.1038/s41598-020-67270-5. Sci Rep. 2020. PMID: 32587307 Free PMC article.
-
CLC-2 single nucleotide polymorphisms (SNPs) as potential modifiers of cystic fibrosis disease severity.BMC Med Genet. 2004 Oct 26;5:26. doi: 10.1186/1471-2350-5-26. BMC Med Genet. 2004. PMID: 15507145 Free PMC article.
-
Glial Chloride Channels in the Function of the Nervous System Across Species.Adv Exp Med Biol. 2021;1349:195-223. doi: 10.1007/978-981-16-4254-8_10. Adv Exp Med Biol. 2021. PMID: 35138616 Free PMC article.
References
-
- Blaisdell C.J., Edmonds,R.D., Wang,X.T., Guggino,S. and Zeitlin,P.L. (2000) pH-regulated chloride secretion in fetal lung epithelia. Am. J. Physiol. Lung Cell Mol. Physiol., 278, L1248–L1255. - PubMed
-
- Byers S., Graham,R., Dai,H.N. and Hoxter,B. (1991) Development of Sertoli cell junctional specializations and the distribution of the tight-junction-associated protein ZO-1 in the mouse testis. Am. J. Anat., 191, 35–47. - PubMed
-
- Cherubini E., Gaiarsa,J.L. and Ben-Ari,Y. (1991) GABA: an excitatory transmitter in early postnatal life. Trends Neurosci., 14, 515–519. - PubMed
-
- Choi M.S. and Cooke,B.A. (1990) Evidence for two independent pathways in the stimulation of steroidogenesis by luteinizing hormone involving chloride channels and cyclic AMP. FEBS Lett., 261, 402–404. - PubMed
-
- Enders G.C. and May,J.J.,II (1994) Developmentally regulated expression of a mouse germ cell nuclear antigen examined from embryonic day 11 to adult in male and female mice. Dev. Biol., 163, 331–340. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

