Sp110 localizes to the PML-Sp100 nuclear body and may function as a nuclear hormone receptor transcriptional coactivator
- PMID: 10913195
- PMCID: PMC86089
- DOI: 10.1128/MCB.20.16.6138-6146.2000
Sp110 localizes to the PML-Sp100 nuclear body and may function as a nuclear hormone receptor transcriptional coactivator
Abstract
The nuclear body is a multiprotein complex that may have a role in the regulation of gene transcription. This structure is disrupted in a variety of human disorders including acute promyelocytic leukemia and viral infections, suggesting that alterations in the nuclear body may have an important role in the pathogenesis of these diseases. In this study, we identified a cDNA encoding a leukocyte-specific nuclear body component designated Sp110. The N-terminal portion of Sp110 was homologous to two previously characterized components of the nuclear body (Sp100 and Sp140). The C-terminal region of Sp110 was homologous to the transcription intermediary factor 1 (TIF1) family of proteins. High levels of Sp110 mRNA were detected in human peripheral blood leukocytes and spleen but not in other tissues. The levels of Sp110 mRNA and protein in the human promyelocytic leukemia cell line NB4 increased following treatment with all-trans retinoic acid (ATRA), and Sp110 localized to PML-Sp100 nuclear bodies in ATRA-treated NB4 cells. Because of the structural similarities between Sp110 and TIF1 proteins, the effect of Sp110 on gene transcription was examined. An Sp110 DNA-binding domain fusion protein activated transcription of a reporter gene in transfected mammalian cells. In addition, Sp110 produced a marked increase in ATRA-mediated expression of a reporter gene containing a retinoic acid response element. Taken together, the results of this study demonstrate that Sp110 is a member of the Sp100/Sp140 family of nuclear body components and that Sp110 may function as a nuclear hormone receptor transcriptional coactivator. The predominant expression of Sp110 in leukocytes and the enhanced expression of Sp110 in NB4 cells treated with ATRA raise the possibility that Sp110 has a role in inducing differentiation of myeloid cells.
Figures


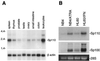



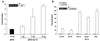
Similar articles
-
Structural and functional heterogeneity of nuclear bodies.Mol Cell Biol. 1999 Jun;19(6):4423-30. doi: 10.1128/MCB.19.6.4423. Mol Cell Biol. 1999. PMID: 10330182 Free PMC article.
-
Identification and characterization of a leukocyte-specific component of the nuclear body.J Biol Chem. 1996 Nov 15;271(46):29198-204. doi: 10.1074/jbc.271.46.29198. J Biol Chem. 1996. PMID: 8910577
-
LYSP100-associated nuclear domains (LANDs): description of a new class of subnuclear structures and their relationship to PML nuclear bodies.Blood. 1996 Aug 15;88(4):1423-6. Blood. 1996. PMID: 8695863
-
Role and fate of PML nuclear bodies in response to interferon and viral infections.Oncogene. 2001 Oct 29;20(49):7274-86. doi: 10.1038/sj.onc.1204854. Oncogene. 2001. PMID: 11704856 Review.
-
Nuclear dots: actors on many stages.Immunobiology. 1997 Dec;198(1-3):307-31. doi: 10.1016/S0171-2985(97)80051-4. Immunobiology. 1997. PMID: 9442402 Review.
Cited by
-
Identification of genetic associations of SP110/MYBBP1A/RELA with pulmonary tuberculosis in the Chinese Han population.Hum Genet. 2013 Mar;132(3):265-73. doi: 10.1007/s00439-012-1244-5. Epub 2012 Nov 6. Hum Genet. 2013. PMID: 23129390
-
Modulation of retinoid signaling by a cytoplasmic viral protein via sequestration of Sp110b, a potent transcriptional corepressor of retinoic acid receptor, from the nucleus.Mol Cell Biol. 2003 Nov;23(21):7498-509. doi: 10.1128/MCB.23.21.7498-7509.2003. Mol Cell Biol. 2003. PMID: 14559998 Free PMC article.
-
Runx3 regulates chondrocyte phenotype by controlling multiple genes involved in chondrocyte proliferation and differentiation.Mol Biol Rep. 2020 Aug;47(8):5773-5792. doi: 10.1007/s11033-020-05646-6. Epub 2020 Jul 13. Mol Biol Rep. 2020. PMID: 32661874
-
The SAND domain protein ULTRAPETALA1 acts as a trithorax group factor to regulate cell fate in plants.Genes Dev. 2009 Dec 1;23(23):2723-8. doi: 10.1101/gad.1812609. Genes Dev. 2009. PMID: 19952107 Free PMC article.
-
Host transcription factor Speckled 110 kDa (Sp110), a nuclear body protein, is hijacked by hepatitis B virus protein X for viral persistence.J Biol Chem. 2017 Dec 15;292(50):20379-20393. doi: 10.1074/jbc.M117.796839. Epub 2017 Oct 18. J Biol Chem. 2017. PMID: 29046350 Free PMC article.
References
-
- Aasland R, Gibson T J, Stewart A F. The PHD finger: implications for chromatin-mediated transcriptional regulation. Trends Biochem Sci. 1995;20:56–59. - PubMed
-
- Alam J, Cook J L. Reporter genes: application to the study of mammalian gene transcription. Anal Biochem. 1990;188:245–254. - PubMed
-
- Bloch D B, de la Monte S M, Guigaouri P, Filippov A, Bloch K D. Identification and characterization of a leukocyte-specific component of the nuclear body. J Biol Chem. 1996;46:29198–29204. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
