A heterozygous mutation of beta-actin associated with neutrophil dysfunction and recurrent infection
- PMID: 10411937
- PMCID: PMC17578
- DOI: 10.1073/pnas.96.15.8693
A heterozygous mutation of beta-actin associated with neutrophil dysfunction and recurrent infection
Abstract
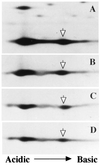
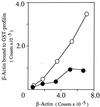
A human disorder caused by mutation in nonmuscle actin has not been reported. We report here a variant of nonmuscle actin in a female patient with recurrent infections, photosensitivity, and mental retardation. She also had abnormalities in neutrophil chemotaxis, superoxide production, and membrane potential response. Two-dimensional PAGE analysis of proteins from neutrophils and other cell types from this patient demonstrated a unique protein spot migrating at 42 kDa with pI shifted slightly to neutral relative to normal beta- and gamma-actin. Digestion peptide mapping and Western blotting showed this spot to be an abnormal actin. A full-length cDNA library was constructed by using mRNA from patient's cells and cDNA encoding the mutant beta-actin molecule was identified by an in vitro translation method. Sequencing of the clones demonstrated a G-1174 to A substitution, predicting a glutamic acid-364 to lysine substitution in beta-actin and eliminating a HinfI DNase restriction site found in normal beta-actin sequence. By HinfI digestion and by sequencing, the mutation in one allele of patient's genomic DNA was confirmed. Though no defect in cell-free polymerization of actin was detected, this defect lies in a domain important for binding to profilin and other actin-regulatory molecules. In fact, the mutant actin bound to profilin less efficiently than normal actin did. Heterozygous expression of mutant beta-actin in neutrophils and other cells of this patient may act in a dominant-negative fashion to adversely affect cellular activities dependent on the function of nonmuscle actin.
Figures

Similar articles
-
Fission yeast Sop2p: a novel and evolutionarily conserved protein that interacts with Arp3p and modulates profilin function.EMBO J. 1996 Dec 2;15(23):6426-37. EMBO J. 1996. PMID: 8978670 Free PMC article.
-
Mutations in beta-actin: influence on polymer formation and on interactions with myosin and profilin.FEBS Lett. 1993 Aug 23;329(1-2):163-70. doi: 10.1016/0014-5793(93)80215-g. FEBS Lett. 1993. PMID: 8354391
-
The binding of mutant actins to profilin, ATP and DNase I.Eur J Biochem. 1992 Oct 1;209(1):171-9. doi: 10.1111/j.1432-1033.1992.tb17274.x. Eur J Biochem. 1992. PMID: 1396697
-
Actin-binding protein complexes at atomic resolution.Annu Rev Biophys Biomol Struct. 1995;24:643-75. doi: 10.1146/annurev.bb.24.060195.003235. Annu Rev Biophys Biomol Struct. 1995. PMID: 7663130 Review.
-
Neutrophil cytoskeletal disease.Int J Hematol. 2001 Aug;74(2):119-24. doi: 10.1007/BF02981993. Int J Hematol. 2001. PMID: 11594510 Review.
Cited by
-
Dynamics of Transcription Regulation in Human Bone Marrow Myeloid Differentiation to Mature Blood Neutrophils.Cell Rep. 2018 Sep 4;24(10):2784-2794. doi: 10.1016/j.celrep.2018.08.018. Cell Rep. 2018. PMID: 30184510 Free PMC article.
-
Mutations and polymorphisms of the skeletal muscle alpha-actin gene (ACTA1).Hum Mutat. 2009 Sep;30(9):1267-77. doi: 10.1002/humu.21059. Hum Mutat. 2009. PMID: 19562689 Free PMC article.
-
De novo mutations in the actin genes ACTB and ACTG1 cause Baraitser-Winter syndrome.Nat Genet. 2012 Feb 26;44(4):440-4, S1-2. doi: 10.1038/ng.1091. Nat Genet. 2012. PMID: 22366783 Free PMC article.
-
Proteomic analysis of colonic mucosal tissue from tuberculous and ulcerative colitis patients.Korean J Physiol Pharmacol. 2012 Jun;16(3):193-8. doi: 10.4196/kjpp.2012.16.3.193. Epub 2012 Jun 26. Korean J Physiol Pharmacol. 2012. PMID: 22802701 Free PMC article.
-
Actin in hair cells and hearing loss.Hear Res. 2012 Jun;288(1-2):89-99. doi: 10.1016/j.heares.2011.12.003. Epub 2011 Dec 13. Hear Res. 2012. PMID: 22200607 Free PMC article. Review.
References
-
- Schleicher M, Andre B, Andreoli C, Eichinger L, Haugwitz M, Hofmann A, Karakesisoglou J, Stockelhuber M, Noegel A A. FEBS Lett. 1995;369:38–42. - PubMed
-
- Drummond D R, Hennessey E S, Sparrow J C. Mol Gen Genet. 1991;226:70–80. - PubMed
-
- Taniguchi S, Sagara J, Kakunaga T. J Biol Chem. 1988;103:707–713. - PubMed
-
- Boxer L A, Hedley-Whyte E T, Stossel T P. N Engl J Med. 1974;291:1093–1099. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

